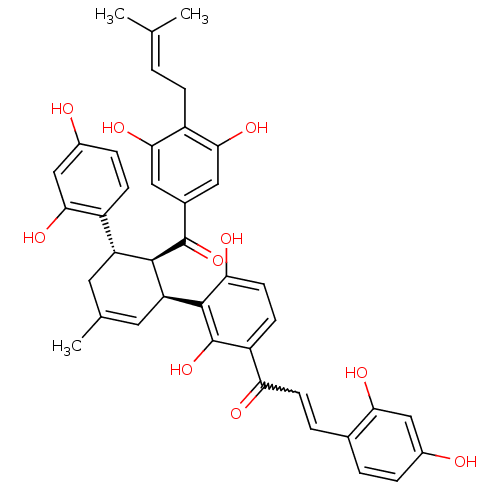
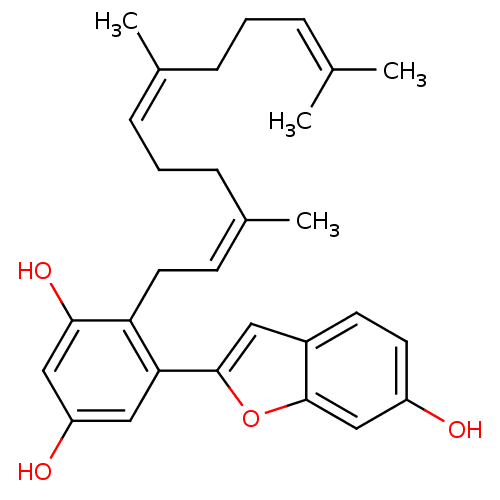
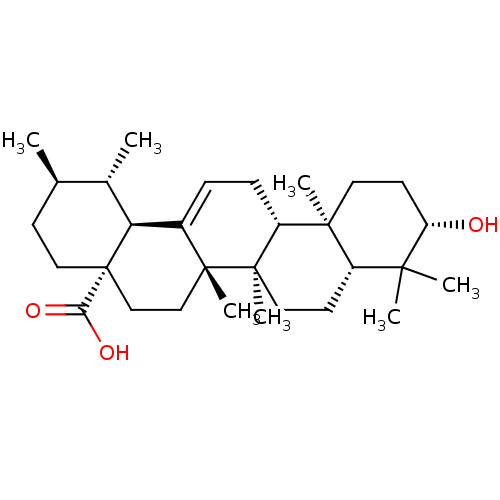
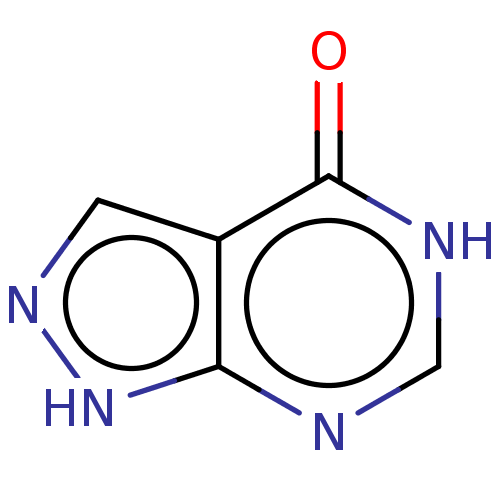
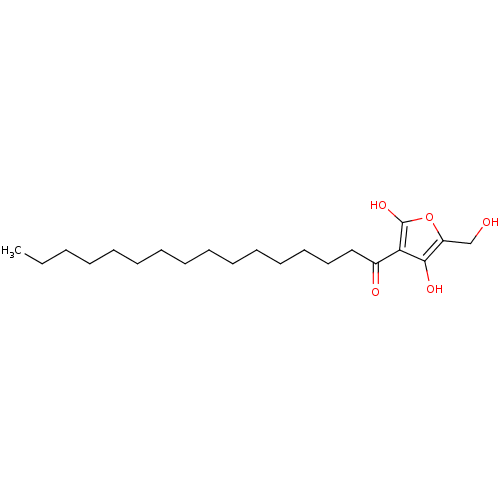
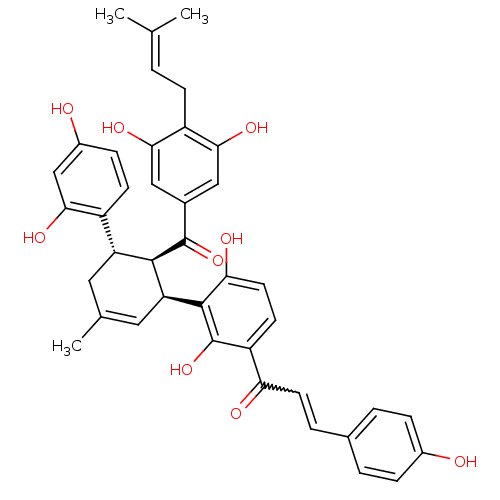
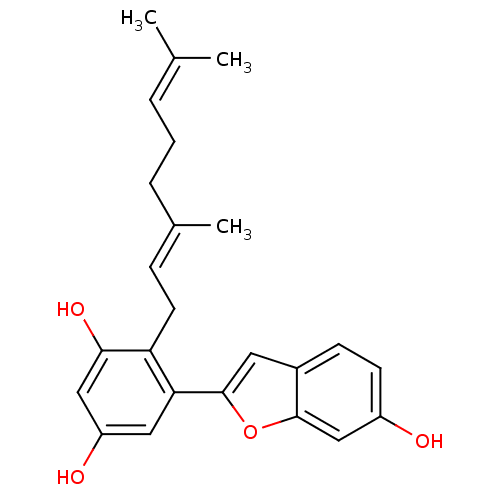
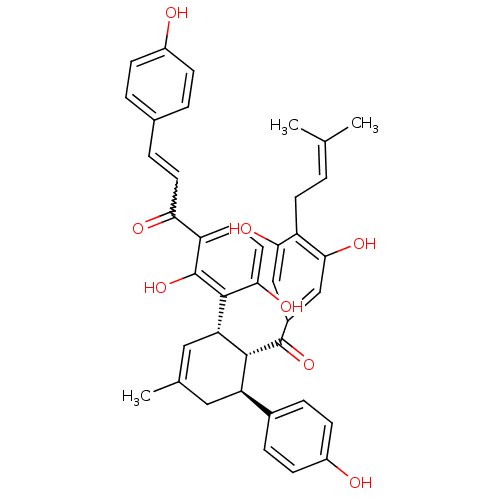
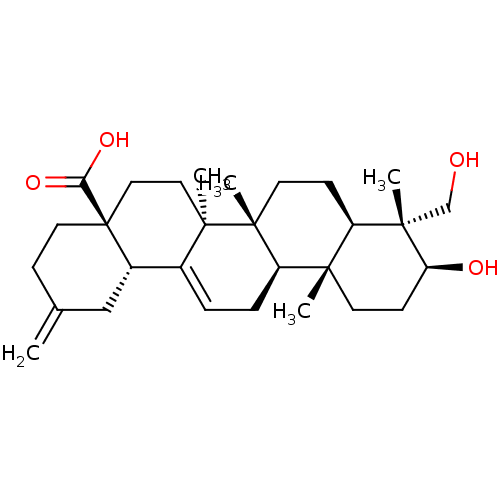
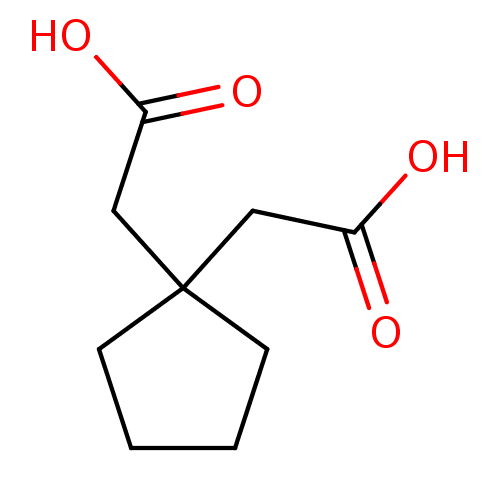
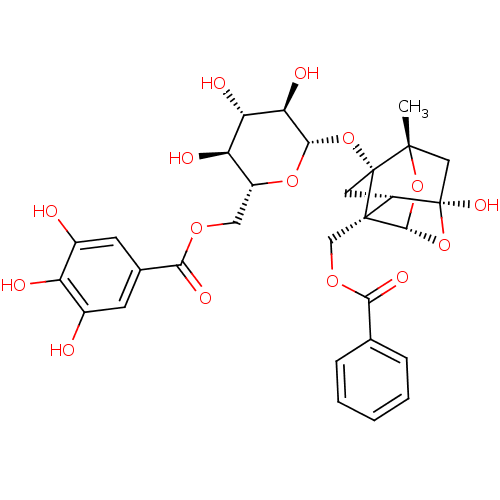
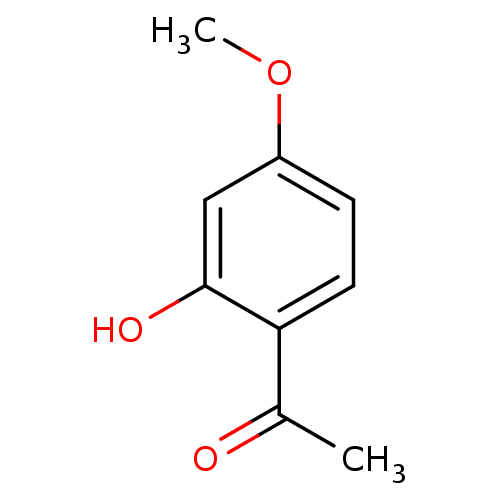
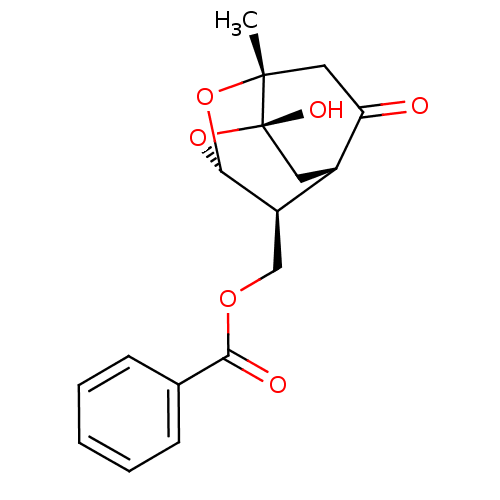
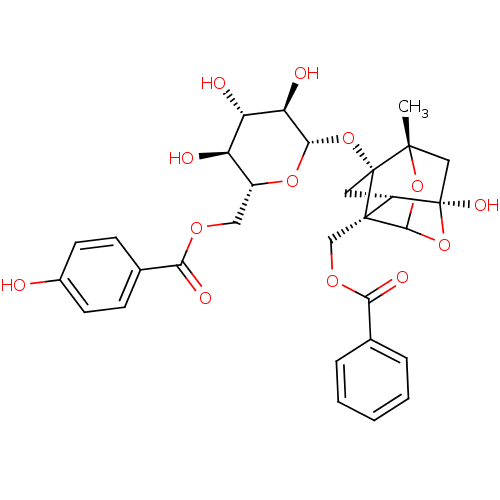
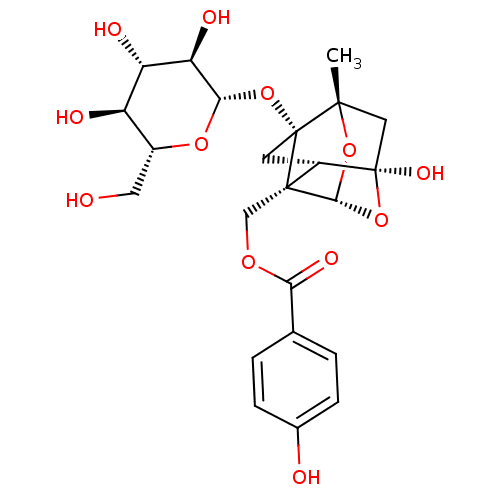
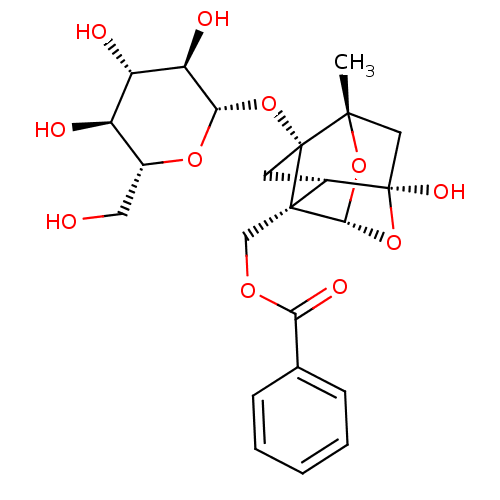
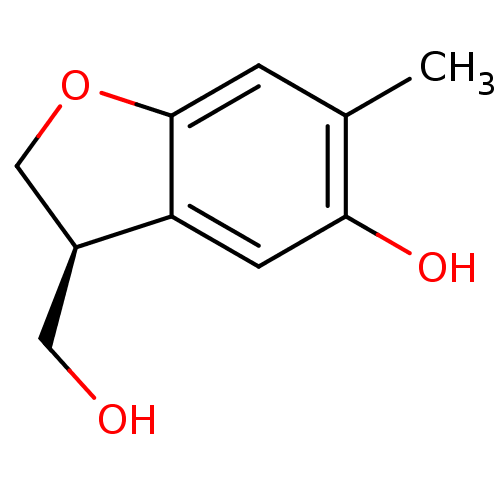
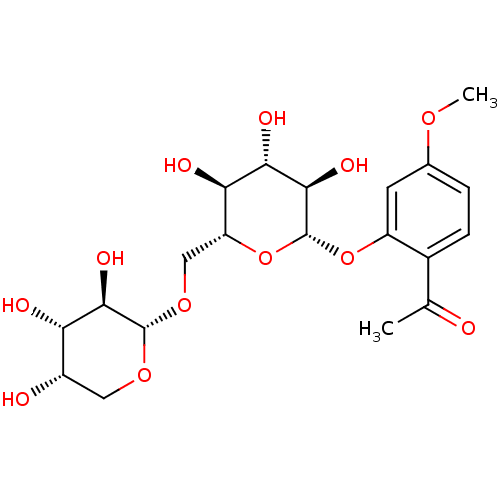
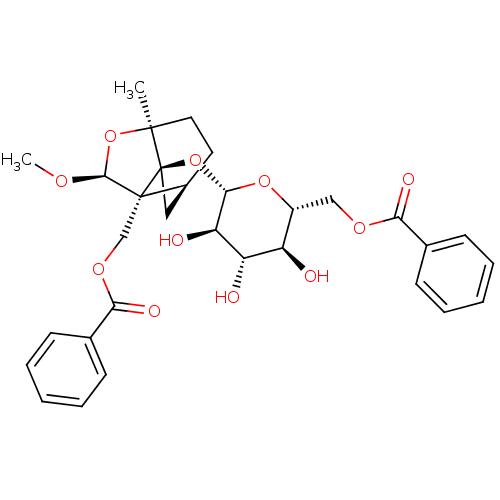
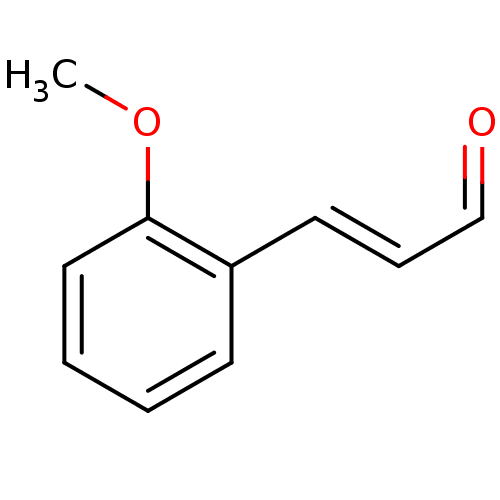
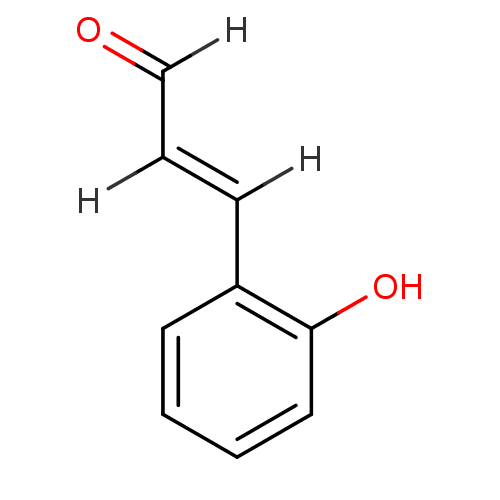
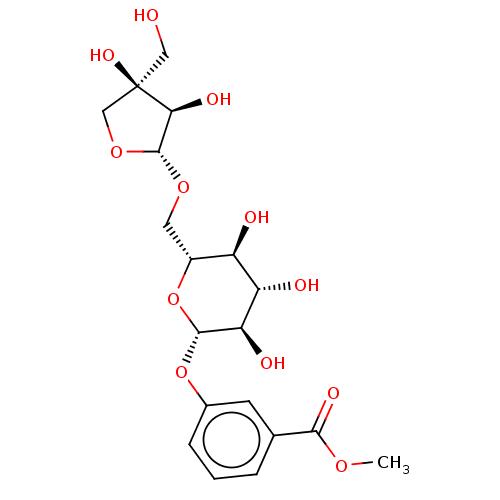
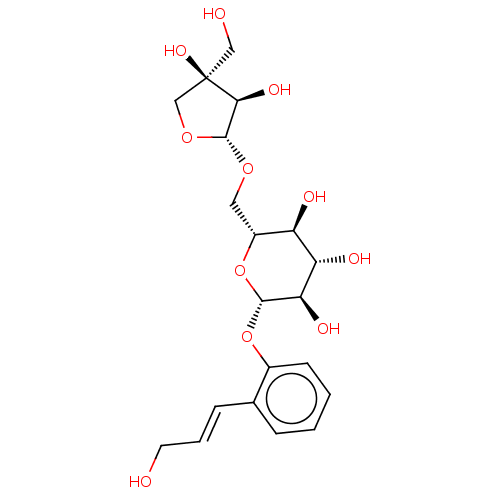
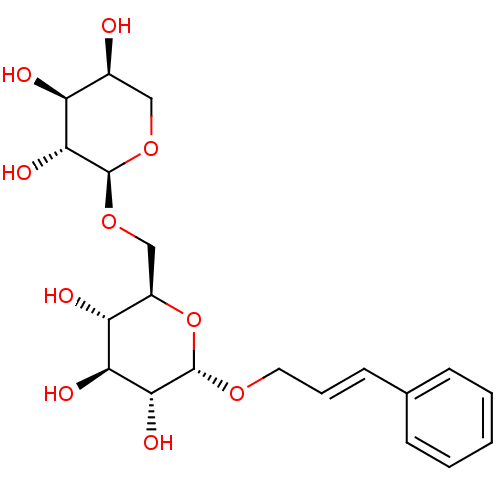
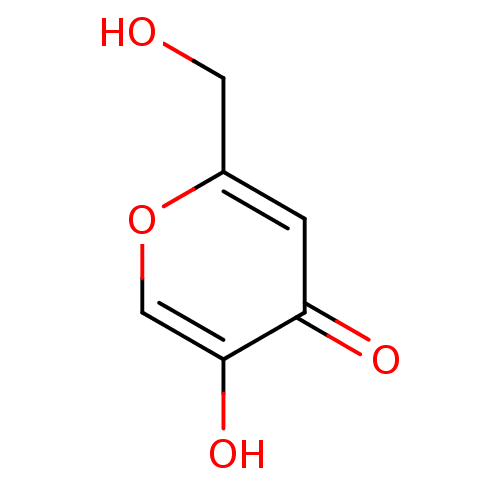
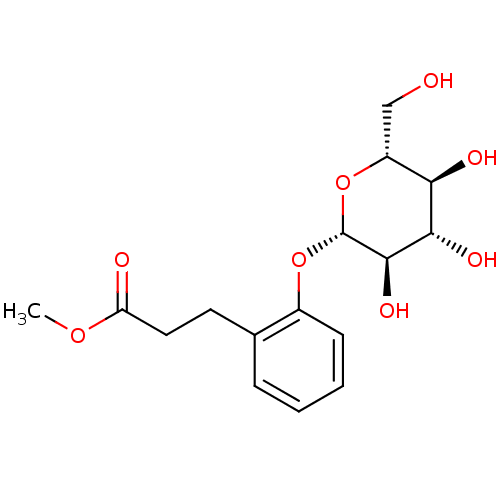
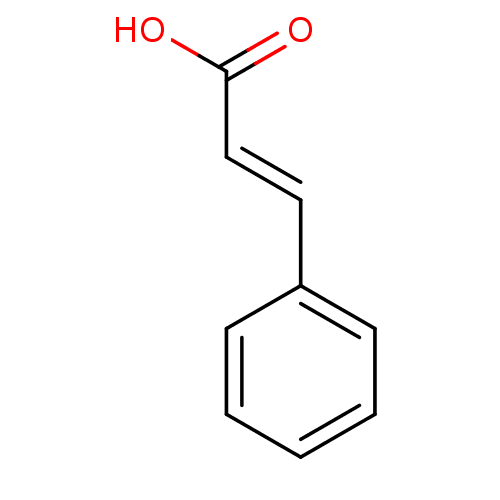
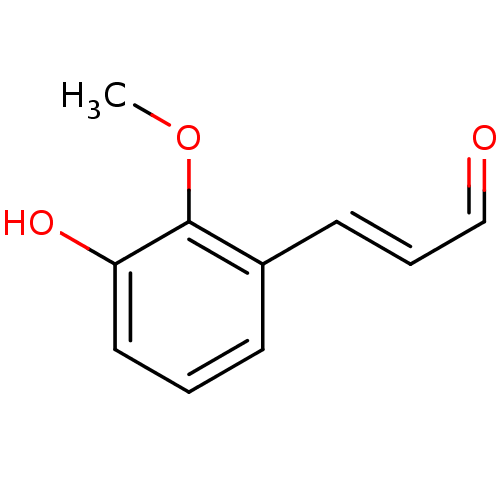
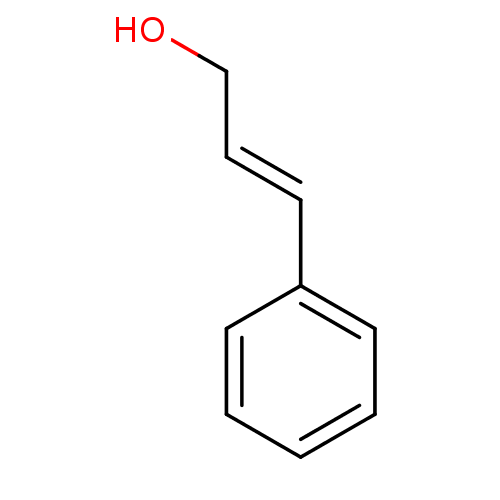
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: 3.89E+3nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 8.20E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 9.20E+3nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 1.14E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Korea Research Institute Of Bioscience And Biotechnology
Curated by ChEMBL
Affinity DataIC50: 1.38E+4nMAssay Description:Inhibition of human recombinant PTP1BMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 2.88E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 3.18E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 3.62E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 4.46E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Rattus norvegicus)
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Sprague-Dawley rat lens alodse reductase by spectrofluorimetryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: 5.90E+4nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: >8.52E+4nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: 8.63E+4nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: 1.01E+5nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: >1.12E+5nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: >1.12E+5nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: >1.16E+5nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 1.40E+5nMAssay Description:Inhibition of mushroom tyrosinase after 10 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: >1.46E+5nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: >1.52E+5nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: 1.75E+5nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: 1.94E+5nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: 2.02E+5nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 2.40E+5nMAssay Description:Inhibition of mushroom tyrosinase after 10 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 2.60E+5nMAssay Description:Inhibition of mushroom tyrosinase after 10 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetXanthine dehydrogenase/oxidase(Homo sapiens (Human))
National Institute Of Medicinal Materials
Curated by ChEMBL
National Institute Of Medicinal Materials
Curated by ChEMBL
Affinity DataIC50: >3.72E+5nMAssay Description:Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 4.10E+5nMAssay Description:Inhibition of mushroom tyrosinase after 10 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 4.20E+5nMAssay Description:Inhibition of mushroom tyrosinase after 10 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 4.70E+5nMAssay Description:Inhibition of mushroom tyrosinase after 10 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 5.20E+5nMAssay Description:Inhibition of mushroom tyrosinase after 10 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 5.70E+5nMAssay Description:Inhibition of mushroom tyrosinase after 10 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Chungnam National University
Curated by ChEMBL
Chungnam National University
Curated by ChEMBL
Affinity DataIC50: 6.30E+5nMAssay Description:Inhibition of mushroom tyrosinase after 10 mins by spectrophotometryMore data for this Ligand-Target Pair